

A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes (see monoisotopic elements), and the average isotopic mass of an isotopic mixture for an element (called the relative atomic mass) in a defined environment on Earth, determines the element's standard atomic weight.

Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the " relative isotopic mass"), is within 1% of the whole number A.Ītoms with the same atomic number but different neutron numbers, and hence different mass numbers, are known as isotopes.

In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.įor an ordinary atom, the sum of the atomic number Z and the neutron number N gives the atom's atomic mass number A. The atomic number can be used to uniquely identify ordinary chemical elements. For ordinary nuclei, this is equal to the proton number ( n p) or the number of protons found in the nucleus of every atom of that element. The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. Both the concept of atomic number and the Bohr model were thereby given scientific credence. Experimental measurement by Henry Moseley of this radiation for many elements (from Z = 13 to 92) showed the results as predicted by Bohr.
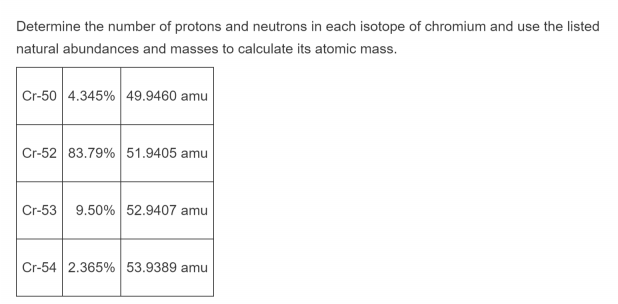
In this model it is an essential feature that the photon energy (or frequency) of the electromagnetic radiation emitted (shown) when an electron jumps from one orbital to another be proportional to the mathematical square of atomic charge ( Z 2). (in other words we reduced 100% to decimal form 1.The Rutherford–Bohr model of the hydrogen atom ( Z = 1) or a hydrogen-like ion ( Z > 1). We will let 6Li = x and 7 Li = 1-x we use 1 – x instead of 100 – x because the small number is easier to work with. Since I don’t know what the percentage are, I will have to use variables.ġ00% of Lithium is determined by these two naturally occurring isotopes. Determine the percent abundance of each isotope.Īw = + + Ħ.94 = + The atomic mass of lithium is 6.94, the naturally occurring isotopes are 6Li = 6.015121 amu, and 7Li = 7.016003 amu. What are the percent abundances of the isotopes? Since the overall atomic weight for copper is not given in the problem, you must look it up in the periodic table to work this solution. If you look in the periodic table you will be able to check that our answer is correct!ģVerify that the atomic mass of magnesium is 24.31, given the followingĪtomic mass= + + ĭetermining the percent abundance of each isotope from atomic mass.Ĭopper exists as two isotopes: 63Cu (62.9298 amu) and 65Cu (64.9278 amu). 10.81amu so, the atomic weight of B = 10.81amu


 0 kommentar(er)
0 kommentar(er)
